Effects of Acid Rain On Fish
Acidity increase has an array of effects onto the individual
fish as well as the population as a whole. Lowered pH levels increase aluminum
levels that are directly toxic to fish. These changes cause chronic stress upon
the fish lowering their body weight (Charles T. Driscoll).
At very low pH (3 or less) levels coagulation of mucous on
gill surfaces and subsequent anoxia would the cause of death, however acidic
levels are rarely this low. At less extreme pH levels (4-5) a disturbance of
normal ion and acid- base balance may be the more likely cause of death. (Carl L. Schofield 1976)
Some species have a greater tolerance to acidic waters. But
those most sensitive face a greater consequence. Often the young are most
vulnerable and most fish eggs won’t hatch at a pH of 5 or less. (U.S
Environment agency).
There has been observed decreased population density and a
shift in size and age structure of fish due to increased acidity. Even a small
increase of fish young fish mortality can be sufficient to decrease population
and eventually lead to extinction. Often the decrease is not recognizable until
the population is close to extinction (Schindler 1988). Spring-spawning fish
face more exposure to a strong acid and aluminum pulse due to snowmelt,
especially since the sensitive young fish are in shallow near shore waters.
(Schindler 1988)
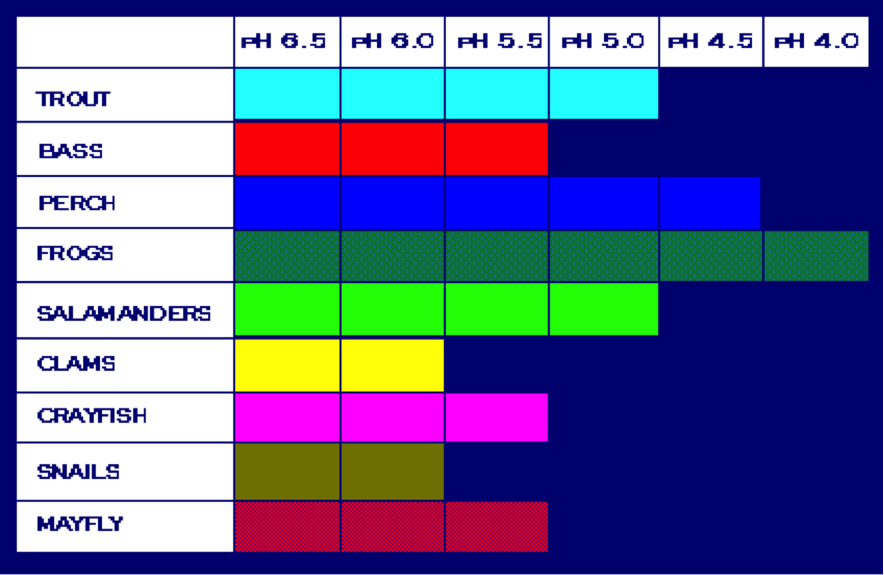
This table shows the pH level tolerance of certain aquatic
species
No comments:
Post a Comment