Acid Snow
Thursday, 20 March 2014
Acid precipitation is any form of
precipitation, including rain, snow, hail, fog, or dew that is high in acid
pollutants, especially sulfuric and nitric acid. It is precipitation with
higher than normal amounts of Nitric and Sulphuric acid causing a lower PH
Level. These higher amounts of acid are due to anthropomorphic systems.
The pH scale is used to measure how basic or
acidic a liquid is. The pH scale ranges from 0 to 14. Bases are from 7 to 14
and acids are found between 0 and 7. The lower the number on the scale the more
acidic the liquid is and the higher the number on the scale the more basic the
liquid is.
This diagram shows the water cycle and how contamination can
lead to acid rain. Acidic gases are released into the atmosphere through car
emissions and industries. Sulphur dioxide and nitrogen oxide are the two main
gases that are released into the atmosphere from factories and nitrogen oxide
is released from the car emissions. These gases are carried by the wind and
they dissolve in rain water to form acid precipitation. Acid precipitation can
occur in many forms and returns to earth in the form of rain, snow, sleet, and
fog.
Acid Snow and Black Snow
Snow is a basin for acids during the winter season by absorbing and storing atmospheric pollutants. As snow falls it collects pollutants in the atmosphere,when it lands it collects pollutants from dry deposition and even turns black ,which is were the term black snow comes from.In the spring the snow melts and turns into runoff and can make its way into streams, rivers and lakes. Acidic episodes are defined as short-term decreases of acid neutralizing capacity or pH that occur during high streamflow associated with rainstorms and snowmelt. Results from the Environmental Results Program have been reported elsewhere and contributed greatly to our understanding of acidic deposition impacts on streams.3-7 Significant acidic episodes were observed in the ERP study streams, and these were attributed to multiple factors including high SO4 . High NO3 levels was responsible for most of the acidity during spring snowmelt. During acidic episodes the stream water was found to be toxic to fish and other aquatic life. Schofield,CL (1977)
Effects of Acid Rain On Fish
Acidity increase has an array of effects onto the individual
fish as well as the population as a whole. Lowered pH levels increase aluminum
levels that are directly toxic to fish. These changes cause chronic stress upon
the fish lowering their body weight (Charles T. Driscoll).
At very low pH (3 or less) levels coagulation of mucous on
gill surfaces and subsequent anoxia would the cause of death, however acidic
levels are rarely this low. At less extreme pH levels (4-5) a disturbance of
normal ion and acid- base balance may be the more likely cause of death. (Carl L. Schofield 1976)
Some species have a greater tolerance to acidic waters. But
those most sensitive face a greater consequence. Often the young are most
vulnerable and most fish eggs won’t hatch at a pH of 5 or less. (U.S
Environment agency).
There has been observed decreased population density and a
shift in size and age structure of fish due to increased acidity. Even a small
increase of fish young fish mortality can be sufficient to decrease population
and eventually lead to extinction. Often the decrease is not recognizable until
the population is close to extinction (Schindler 1988). Spring-spawning fish
face more exposure to a strong acid and aluminum pulse due to snowmelt,
especially since the sensitive young fish are in shallow near shore waters.
(Schindler 1988)
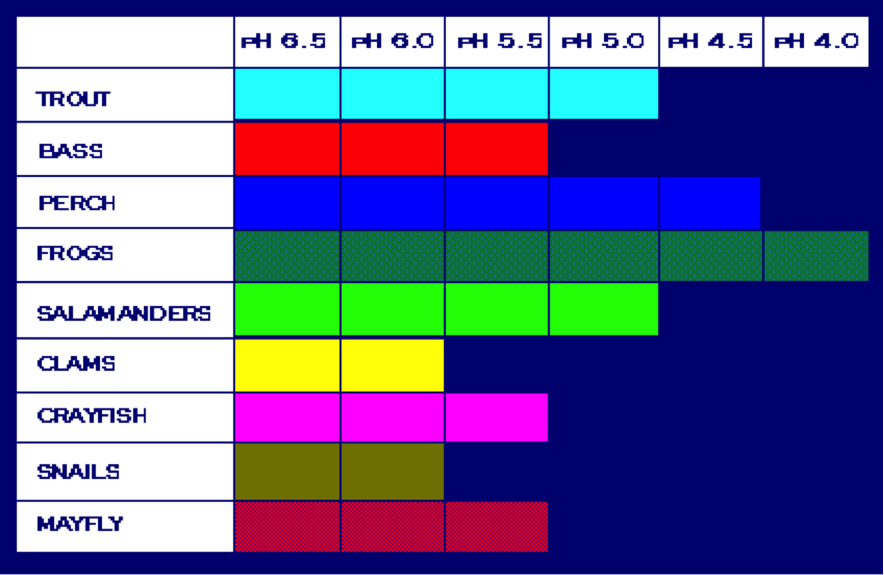
This table shows the pH level tolerance of certain aquatic
species
Carl L. Schofield. Acid Precipitation: Effects on Fish. Ambio , Vol. 5, No. 5/6 (1976) , pp. 228-230. Springer on behalf of Royal Swedish Academy of Sciences.
CHARLES T. DRISCOLL, GREGORY B. LAWRENCE, ARTHUR J. BULGER, THOMAS J. BUTLER, CHRISTOPHER S. CRONAN, CHRISTOPHER EAGAR, KATHLEEN F. LAMBERT, GENE E. LIKENS, JOHN L. STODDARD, and KATHLEEN C. WEATHERS. Acidic Deposition in the Northeastern United States: Sources and Inputs, Ecosystem Effects, and Management Strategies. BioScience , Vol. 51, No. 3 (March 2001) , pp. 180-198. University of California Press.
Schindler, David W. "Effects of acid rain on freshwater ecosystems." Science(Washington) 239.4836 (1988): 149-157.
U.S Environmental Agency. Effects of Acid Rain - Surface Waters and Aquatic Animals. December 4, 2012. EPA.
Ottawa River
On August 8TH, 2013 hundreds of fish washed up
dead on shore of the Ottawa River. Ottawa’s health officials are currently
investigating the death of these fish.
This could possibly be the result of increase acidity in The Ottawa River,
which could be due to acid precipitation or runoff. One of the main species of fish found death is
Catfish who cannot survive in a pH level of lower than 4.
News, C. (2006, August 15). Hundreds of dead fish wash up along Ottawa River. CBCnews. Retrieved February 14, 2014, from http://www.cbc.ca/news/canada/ottawa/hundreds-of-dead-fish-wash-up-along-ottawa-river-1.612913
Subscribe to:
Comments (Atom)